To find out more about Cerulean’s ongoing clinical trials evaluating CRLX101, please visit www.clinicaltrials.gov
CRLX101 is comprised of the high potency anti-tumor agent camptothecin coupled to a cyclodextrin based polymer that self-assembles into nanoparticles of consistent size and other physical attributes.
Below is a schematic representation of CRLX101
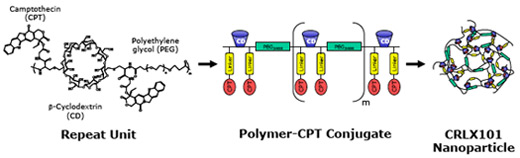
CRLX101 provides clinical validation of the CDP technology improving the tolerability of the parent drug camptothecin. Results from the Phase 1 clinical study of CRLX101 have shown that it has a favorable safety profile in patients with advanced cancer. Combining camptothecin’s potency and Cerulean’s nanopharmaceutical design features, we believe CRLX101 has the potential to kill tumor cells while minimizing the side effects typically associated with chemotherapy treatment.
Phase 2a clinical studies with advanced cancer patients are presently being carried out at the maximum tolerated dose.